Cancer Therapy Vol 7, 302-304, 2009
A phase II study of anastrozole plus the pineal anticancer hormone melatonin in the metastatic breast cancer women with poor clinical status
 Research Article (download PDF version)
Research Article (download PDF version)
Paolo Lissoni1*, Giuseppe Di Fede1, Antonio Battista2, Giusy Messina1, Remo Egardi1, Fernando Brivio3, Franco Rovelli1, Massimo Colciago4, Giuseppe Brera5
1 Institute of Biological Medicine, Milan
2 Azienda Sanitaria locale 2, Avellino;
3 Surgery Division, Bassini Hospital, Cinisello,Milan
4 I.N.R.C.A, Casatenovo, Lecco, Italy
5 Ambrosian University, Milan, Italy
dr. Paolo Lissoni
*Correspondence: Dr. Paolo Lissoni, Divisione di Radioterapia Oncologica, Ospedale S.Gerardo, 20052 Monza, Milano, Italy; Fax: +390392332284, E-mail: p.lissoni@hsgerardo.org
Key words: Anastrozole, breast cancer, melatonin, pineal gland
Abbreviations: melatonin, MLT; estrogen receptor, ER;
Received: 9 March 2009; Revised 1 April 2009;
Accepted: 13 April 2009; electronically published: 28 May 2009
Summary
The recent advances in the psychoneuroendocrinology have suggested the possibility to modulate tumor hormone dependency through a neuroendocrine approach. In particular, it has been proven that the pineal neurohormone melatonin (MLT) may stimulate estrogen receptor (ER) expression in breast cancer cells and inhibit the aromatase activity. On this basis, a study was planned to evacuate the efficacy of a concomitant treatment with the aromatase inhibitor anastrozole plus MLT in metastatic breast cancer. The study included 14 metastatic breast cancer women of poor clinical conditions with ER positive or unknown. Both anastrozole and MLT were given orally at a dose of 1 mg at noon and of 20 mg in the evening, respectively. The clinical response consisted of complete response in 2 and partial response in 6 patients. Then, an objective tumor regression was achieved in 8/14 (57%) patients, with a median duration of 26 months. No neoplastic cachexia occurred on treatment. This preliminary study shows that a neuroendocrine strategy with anastrozole plus the pineal hormone MLT may represent a new effective and well tolerated regimen in the treatment of metastatic breast cancer women, including those with poor clinical status, with therapeutic results apparently superior to those reported in the literature with the only aromatase inhibitor. Then, these results would justify further randomized studies of aromatase inhibitors with or without a concomitant administration of MLT, in an attempt to establish whether the pineal hormone may enhance the efficacy of the aromatase inibibitors in the treatment of human advanced breast cancer.
I. Introduction
Recent experimental studies have demonstrated that the hormone dependency is at least in part under a psychoneuroendocrine regulation (Cos et al, 2008;Grant et al, 2009). In particular, it has been shown that the pineal hormone melatonin (MLT), whose anticancer properties have been well demonstrated (Bartsch et al, 1981; Maestroni, 1993; Reiter et al, 2002), may in vitro stimulate estrogen receptor (ER) expression on breast cancer cell lines (Molis et al, 1995). Therefore, the hormone dependency of breast cancer cells would not depend only on intrinsic characteristics of cancer cells themselves, but also on host neuroendocrine regulation of tumor cell proliferation and differentiation (Bartsch et al, 2000). Moreover, cancer progression has been proven to be associated with pineal alterations, consisting of a progressive decline in MLT nocturnal production. (Maestroni, 1993). Therefore the advanced cancer would require a substitutive endocrine therapy with MLT (Bartsch et al, 1981; Maestroni, 1993). Previous preliminary clinical studies had already suggested that the concomitant administration of the pineal hormone MLT may apparently increase the efficacy of tamoxifen therapy in the treatment of metastatic breast cancer (Lissoni et al, 1995). Moreover, experimental studies have shown that the activity of aromatase enzyme, which is responsible for the peripheral production of estrogens from testosterone (Bagatell et al, 1994), is under a light/dark circadian rhythm (Bhatnagar et al, 1992). Because of the fundamental role of the pineal hormone MLT in the regulation of the daily photoperiod (Bartsch et al, 1981), it is possible to hypothesize that MLT may be involved in the control of the aromatase activity. In fact, recent studies have demonstrated an inhibitory action of MLT on the aromatase activity (Cos et al, 2005). This finding could reserve a prosiming application in the treatment of both early and advanced breast cancer. This statement is justified by the fact that the aromatase inhibitors represent a new class of agents in the endocrine treatment of breast cancer Plourde et al, 1994), with a potential efficacy superior to that achieved by the previous hormonal therapies with anti-estrogens, such as tamoxifene, even though tumor response rate obtained by the aromatase inhibitors are generally not greater than 40%. On this basis, a phase II study was planned in an attempt to evaluate the efficacy of a neuroendocrinotherapeutic regimen consisting of a concomitant administration of the aromatase inhibitor anastrozole and the pineal hormone MLT in metastatic breast cancer women with poor clinical conditions.
II. Materials and methods
The study included 14 consecutive metastatic breast cancer women (median age: 72 years, range 51-82), who were followed at Biological Medicine Institute in Milan, or at Health Local Unit 2 of Avellino, from Feb. 2002 to Sept. 2003. Eligibility criteria were, as follows: histologically proven metastatic breast cancer, measurable lesions, ER positive or unknown, no ability to tolerate chemotherapy because of age, low performance status (PS), important clinical illnesses other than cancer and/or heavy chemotherapeutic pre-treatments, no previous endocrine therapies for the metastatic disease, no double tumor and life expectancy less than 1 year. Previous heavy chemotherapeutic treatment consisting of at least 3 chemotherapeutic lines was made in 11/14 (79 %) patients. Dominant metastasis sites were, as follows: soft tissues:1; bone:1; lung:7 (neoplastic lymphangitis:2); liver:1; lung + liver:1; bone marrow:3. Time-span since first diagnosis of the primary tumor was 44 months (31-66 months). All patients had an acceptable social conditions. The minimum and median follow-up periods were 60 months and 72 months respectively. In all patients, in the case of disease progression, at least to other endocrine therapeutic lines with other aromatase-inhibitors were planned. The experimental protocol, wich was approved by the Health Direction of Biological Medicine Institute of Milan, was explained to each patient and informed consent was obtained. The treatment consisted of anastrozole at a dose of 1 mg/day orally at noon, plus MLT at 20 mg/day orally in the evening, generally half-hour before sleeping, to correct cancer progression-related decline in MLT night secretion (10). Patients were considered to be evaluable when they were treated for at least 3 consecutive months. The clinical response was evaluated according to WHO criteria. Complete response (CR) was the complete disappearance of all neoplastic lesions for at least 1 month. Partial response (PR) was a reduction greater than 50 % of the sum of all neoplastic lesions, for at least 1 month. Stable disesase (SD) was no increase or decrease greater tha 25 % of tumor volume. Progressive Disease (PD) was an increase in tumor volume greater than 25 % or the appearance of new neoplastic lesions. PS was assessed according to Karnofsky’s score, consisting of the evaluation of the quality of life in relation to patient activity and bed-rest period. ER was positive in 10 and unknown in the remaining 4 patients. The median PS was 80% (range 70-100). Data were statistically evaluated by the chi-square test and the Student’s t test, as appropriate.
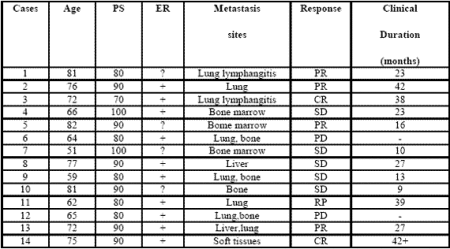
Table 1: Clinical characteristics of metastatic breast cancer women and their clinical response (WHO criteria) to a neuroendocrine regimen consisting of anastrozole plus the pineal hormone melatonin.
III. Results
All patients were fully evaluable for the clinical response. The clinical characteristics of patients and their individual clinical response to the treatment are reported in Table 1. As reported, a complete response (CR) was achieved in 2/14 (14%) (soft tissues:1; lung lymphangitis:1). A partial response (PR) was obtained in other 6/14 (43%) (bone:1; lung:3; liver:1; bone marrow:1). Then, an objective tumor response (CR + PR) was reached in 8/14 (57%) patients. The median duration of response was 26 months (range 9-42 months). A stable disease (SD) was seen in other 4/14 (29%), with a median duration of 25 months (range 10-27). Therefore, a disease-control (DC:CR + PR + SD) was achieved in 12/14 (86%) patients, whereas the remaining 2/14 (14%) patients had a progressive disease (PD). No significant difference in tumor response rate was observed between patients with positive or unknown ER ( 6/10(60%) vs 2/4(50%) ). An overall survival at 1 year and at 3 year was achieved in 11/14 (79 %) and in 5/14 (36 %) patients, respectively. Moreover, 3/14 (21%) patients were still alive at 5 years. The treatment was well tolerated in all patients.
Moreover, most patients experienced a relief of asthenia under the treatment and in no patient the neoplastic cachexia occurred. Finally, an evident increase in PS mean values was achieved under treatment, even though it did not reach the statistical significance (86 ±5 vs 93 ± 4, mean ± SE).
IV. Discussion
The results of this preliminary phase II study, by showing a percentage of 1-year survival greater than 70% in patients with live expectancy less than 1 year, would suggest that a neuroendocrine regimen consisting of the aromatase inhibitor anastrozole plus the pineal neurohormone MLT may represent a new effective therapeutic strategy in the treatment of metastatic breast cancer women, also in patients with poor clinical conditions, who would not be able to tolerate the most aggressive therapies. The concomitant administration of the pineal hormone would seem to enhance the efficacy of the aromatase inhibitor in terms of objective tumor regressions with respect to the results commonly reported in the literature with the only aromatase inhibitor (Plourde et al, 1994), which are generally lower than 40%.
The time to progression would seem to be apparently increased by the concomitant treatment with MLT.This finding is not surprising, since MLT could enhance the therapeutic anticancer acitivity of the aromatase inhibitors by either exerting direct antiproliferative antitumor effects (Bartsch et al, 1981; Maestroni, 1993; Reiter et al, 2002), or further inhibiting the aromatase activity by acting on gene and oncogene expression (Molis et al,1995; Cos et al, 2005). In addiction, MLT appeared to stimulate ER expression of breast cancer lines, by transforming ER negative into ER positive breast cancer, as observed in experimental conditions (Danforth et al, 1983).
Since the prognosis of ER positive breast cancers is clearly better than that of ER negative ones, MLT could per se improve the clinical couse of mammary tumors. Finally, because of its interesting therapeutic efficacy as a supportive care (Reiter et al, 2002), MLT would be responsible for the evident improvement in the relief of asthenia and in preventing the occurrence of the neoplastic cachexia. On the other hand, because of the inhibitory effect of MLT (Grant et al, 2009; Reiter et al, 2002) on cancer cell proliferation, the anticancer activity of this polyendocrine regimen would be due not only to an indirect effect, depending on a diminished estrogen production following aromatase enzyme inhibition, but also on a direct inhibition of cancer cell growth, due to MLT itself. Therefore, the results of this preliminary study may justify further clinical randomized investigations with the only aromatase inhibitor versus the concomitant treatment with MLT, in an attempt to confirm the ability of the pineal hormone to enhance the antitumor properties of the aromatase inhibitors in the treatment of metastatic breast cancer women with poor clinical conditions.
References
Bagatell CJ, Dahl KD, Bremner WJ (1994). The direct pituitary effect of testosterone to inhibit gonadotrophin secretion in men is partially mediated by aromatization to estradiol. J Androl 15:15-21.
Bartsch C, Bartsch H (1981) effect of melatonin on experimental tumors under different photoperiods and times of administration. J Neural Transm 52:269-279.
Bartsch H, Buchberger A, Franz H,Bartsch C,Maidonis I,Mecke D, Bayer E (2000) Effect of melatonin and pineal extracts on human ovarian and mammary tumor cells in a chemosensitivity assay. Life Sci 67:2953-2960.
Bhatnagar AS, Muller P, Schenkel L, Trunet PF, Beh I, Schieweck K. (1992). Inhibition of oestrogen biosynthesis and its consequences on gonadotrophin secretion in the male. J of Steroid Biochemistry and Molecular Biology;41:1021-1027
Cos S, Martinez-Campa C,Mediavilla MD, Sanchez-Barcelo E (2005). Melatonin modulates aromatase activity in MCF-7 human breast cancer cells. J Pineal Res 7:136-142.
Cos S, Gonzales A, Martinez-Campa C, Mediavilla MD, Alonzo-Gonzales C, Sanchez-Barcelo EJ (2008): Melatonin as a selective estrogen enzyme modulator. Curr Cancer Drug Targets. Dec 8(8): 691-702.
Danforth DN, Tamarkin L, Lipmann LE: (1983) Melatonin increase oestrogen receptor binding activity of human breast cancer cells. Nature, 305:323-325.
Grant S.G, Melan MA, Latimer JJ, Witt-Enderby PA (2009) : Melatonin and Breast cancer : cellular mechanism, clinical studies and future perspective. Expert Rev Mol Med 11:e5
Lissoni P, Barni S, Meregalli S, Fossati V, Cazzaniga M, Esposti D, Tancini G (1995) Modulation of cancer endocrine therapy by melatonin:a phase II study of tamoxifen plus melatonin in metastatic breast cancer patients progressing under tamoxifen alone. Br J Cancer 71:854-856.
Maestroni GJM (1993) The immunoneuroendocrine role of melatonin. J Pineal Res 14:1-10.
Molis TM, Spriggs LL,Jupiter Y,Hill SM (1995) Melatonin modulation of estrogen-regulated proteins, growth factors,and protooncogenes in human breast cancer. J Pineal Res 18:93-103.
Plourde PV, Dyroff M, Dukes MD (1994) Arimidex: a potent and selective fourth generation aromatase inhibitor. Breast Cancer Res Treat 30:103-111.
Reiter RJ, Tan DX, Sainz RM, Mayo JC, Lopez-Burillo S. (2002) Melatonin:reducing the toxicity and increasing the efficacy of drugs. Pharm Pharmacol 54:1299-1321