Modulation of the anticancer immunity by natural agents: inhibition of T regulatory lymphocyte generation by arabinoxylan in patients with locally limited or metastatic solid tumors
 Research Article (download PDF version)
Research Article (download PDF version)
Paolo Lissoni1,*, Giusy Messina1, Fernando Brivio2, Luca Fumagalli2, Luigi Vigoré3, Franco Rovelli3, Luisa Maruelli4, Mauro Miceli4, Paolo Marchiori4, Giorgio Porro1, Michael Held5, Giuseppe di Fede6, Toshi Uchiyamada7
1 Division of Radiation Oncology, San Gerardo Hospital, Milan, Italy
2 Division of Surgery, San Gerardo Hospital, Milan, Italy
3 Laboratory of Immunomicrobiology, San Gerardo Hospital, Milan, Italy
4 Natur-Spiritual, Milan, Italy
5 Biological Medicine Center, Rome, Italy
6 Institute of Biological Medicine, Milan, Italy
7 Daiwa Pharmaceuticals, Tokyo, Japan
dr. Paolo Lissoni
*Correspondence: Dr. Paolo Lissoni, Divisione di Radioterapia Oncologica, Ospedale S.Gerardo, 20052 Monza, Milano, Italy; Fax: +390392332284, e-mail: p.lissoni@hsgerardo.org
Key words: Anticancer immunity, arabinoxylan, immunostimulation, T regulatory lymphocytes
Abbreviations: interleukin 10, (IL-10); interleukin 6, (IL-6); interleukin-2, (IL-2); interleukn 12, (IL-12); NK cells, (CD16+CD56+); T cytotoxic lymphocytes, (CD8+); T helper lymphocytes, (TH), (CD4+); T lymphocites, (CD3+); Transforming growth factor beta, (TGF-β) T-regulatory lymphocytes, (T-reg), (CD4+CD25+)
Received: 30 September 2008; Revised: 1 November 2008
Accepted: 17 November 2008; electronically published: December 2008
Summary
In the last years, several immunomodulating antitumor agents have demonstrated in the nature, particularly from Aloe plant and rice bran. However, the major problem concerning the natural antitumor agents is to define their immune mechanisms of action in relation to the more recent advances in tumor immunobiology. At present, the main cause responsible for the lack of an effective antitumor response in advanced cancer patients is belived to be represented by the generation of a subtype of T helper lymphocytes (CD4+) with suppressive activity on anticancer immunity, the so-called T regulatory lymphocytes (T reg), which may be clinically identified as CD4+CD25+ cells. On this basis, a study was planned to evaluate the effect of rice bran extract arabinoxylan on T reg cell count and percentage in solid tumor patients in relation to the various lymphocyte subpopulations. The study included 22 evaluable cancer patients, 16 of whom had an untreatable metastatic solid tumor. Arabinoxylan was given orally at a dose of 2000 mg/day for the first month, followed by a dose of 1000 mg/day for the next month. In each patient we evaluated by monoclonal antibodies the absolute number of lymphocytes, T lymphocytes (CD3+), T helper (TH) lymphocytes (CD4+), T cytotoxic lymphocytes (CD8+), NK cells (CD16+CD56+), T reg lymphocytes (CD4+CD25+) and TH/T reg ratio before and after 2 months of therapy. No substantial change occurred on therapy in the mean number of lymphocytes, CD3+, CD8+ and NK cells. On the other hand, the mean number of TH cells increased, whereas that of T reg cell decreased on treatment, even though none of these differences was statistically significant. On the contrary, TH/T reg mean ratio significantly enhanced after arabinoxylan therapy. In addition to its previously demonstrated stimulatory action on NK function, this study shows that arabinoxylan may inhibit the production of T reg cells, which are responsible for cancer-related immunosuppression, with a following improvement in the anticancer immunity. If further studies will confirm these results, arabinoxylan could be successfully associated with chemotherapy to induce not only a cytotoxic destruction of cancer cells, but also an improvement in the immune status.
I. Introduction
The recent advances in the definition of the mechanisms responsible for tumor progression have suggested the possibility to control cancer growth not only trough chemotherapy-induced cancer cell destruction, but also by stimulating the anticancer immunity. In addiction to the exisence of endogenous antitumor molecules, several agents capable of stimulating the anticancer immunity have alsso isolated from plants. However, the immunomodulatory effects of most natural immunomodulating agents need to be better investigated in an attempt to establish their mechanisms of action in relation to the most recent discoveries concerning the physiopathology of the anticancer immunity. At present, Aloe extracts (Lissoni et al, 1998) and arabinoxylan extract from rice bran (Ghoneum and Jewett, 2000) would represent some of the potential natural agents which could be utilized in the complementary therapy of human neoplasms. Today, it is known that the antitumor immune response is the end-result of several interactions involving cytokines and immune cells, provided by stimulatory or suppressive effects on the anticancer immunity (Atzpodien and Kirchner, 1990; Rosenberg, 1992). Therefore, the lack of an effective anticancer immune response in most cancer patients with advanced disease would simply depend on the prevalence of immunosuppressive mechansisms with respect to the immunostimulatory ones (Atzpodien and Kirchner, 1990). The anticancer immunity is mainly activated by T helper-type 1 lymphocytes by releasing IL-2 (Whittington and Faulds, 1993), and by dentritic cells, which act as antigen-presenting cells producing IL-12 (Banks et al, 1995), T cytotoxic lymphocytes and NK-LAK system, which are involved in the induction of the antigen-dependent and antigen-independent cytotoxicity, respectively (Atzpodien and Kirchner, 1990). Therefore, IL-2 and IL-12 would represent the main anticancer cytokines in humans. On the contrary, the suppression of the anticancer immune response is mediated by several cytokines, namely IL-10 (Moore et al, 1993), IL-6 (Matsuda and Hirano, 1990) and TGF-β (Shevach, 2002). Recently, however, it has been demonstrated that the various endogenous suppressive factors would exert their inhibitory immune effect through a common end-mechanism, consisting of the generation of a subtype of T helper lymphocytes (CD4+cells), provided by a fundamental suppressive activity on the anticancer immunity, the so-called T regulatory lymphocyte (T reg) (Dieckmann et al, 2001), which at present seems to constitute the main mechanism responsible for cancer-related immunosuppressive status. T reg cells may be identified by the simultaneous expression of the alpha-chain of IL-2 receptor (CD25) and CD4 antigen (Dieckmann et al, 2001). Then, T reg cells may be clinically recognized as CD4+CD25+ lymphocytes. Therefore, each eventual natural immunomodulating agent would have to be investigated in relation to its possible effect on T reg generation since, at least from a theoretical point of view, each natural agent capable of counteracting T reg activity could positively influence the prognosis of the neoplastic disease by improving the efficacy of the anticancer immune response. Moreover, our previous preliminary studies have suggested that the percentage of T reg cells with respect to the total number of T helper cells, as expressed as CD4/CD4CD25 ratio, may represent an optimal synthetic immune index to investigate the functional status of the anticancer immunity in the single cancer patient, by representing the synthesis of the actions of the great number of immunostimulating and immunosuppressive factors involved in the modulation of the anticancer immunity (Dieckmann et al, 2001). Within the great number of natural agents derived from plants and potentially usefull to be employed in the complementary therapy of cancer, arabinoxylan would seem to represent one of the potential natural agent, because of its efficacy in improving the clinical status of cancer patients (Ghoneum and Jewett, 2000; Ghoneum and Gollapudi, 2005; Markus et al, 2006; Ghoneum et al, 2007). The immunomodulating properties of this nautral substances extracted from plants have been confirmed by experimental studies, but unfortunately most experiments have been limited to the investigations of they effects on non-specific immune parameters for the anticancer immunity, such as NK cell cytotoxicity. In contrast, since reg cells play a fundamental role in suppressing the generation of the anticancer immunty, each potential antitumor immunomodulatory natural substances, would have to be investigated also in relation to their eventual influence on T reg cell system. On the basis of the recent discoveries in tumor immunobiology (Dieckmann et al, 2001; Shevach, 2002), a study was planned to investigate the possible influence of arabinoxylan on both absolute number of T reg cells and their ratio with respect to the total CD4+ T cells in a group of solid tumor patients, affected by locally limited or metastatic disease.
II. Materials and methods
The study included 24 consecutive patients, 18 of whom had a metastatic solid tumor, which did not respond to the conventional anticancer chemotherapies and for whom no other effective standard treatment was available, while the remaining 6 patients had been surgically treated for a locally limited neoplasm. Patients were followed at Biological Medical Institute of Milan and the protocol was approved by the Director of the Institute. Eligibility criteria were, as follows:histologically proven locally limited or metastatic solid tumor, no double tumor, no chronic therapy with corticosteroids because of their immunosuppressive effects and no concomitant treatment with other immunomodulating agents,such as interferons,interleukins and monoclonal antibodies. At the time of the start of arabinoxylan therapy, patients with untreatable metastatic cancer were under treatment with the only supportive care, consisting of anti-inflammatory agents for pain, anti-dopaminergic drugs for nausea and vomiting and with the pineal hormone melatonin for the therapy of the neoplastic cachexia (Banks et al, 1995). Patients were considered as fully evaluable when they had received arabinoxylan therapy for at least 2 consecutive months. Arabinoxylan was given orally at a dose of 1000 mg twice/day for the first month, followed by a dose of 1000 mg/day for the next month. Arabinoxylan was supplied by DAIWA Pharmaceutical (Tokyo, Japan). It was derived from rice bran treated enzymatically with an extract of the shiitake mushrooms. It is a polysaccharide containing β-1,4-xylopironase hemicellulose, commercially available and known as Biobran. For the immune investigation, venous blood samples were collected in the morning after an overnight fast before the onset of arabinoxylan therapy and after 2 consecutive months of treatment. In each blood sample, we evaluated the absolute number of total lymphocytes, T lymphocytes (CD3+), T helper (TH) lymphocytes (CD4+), T cytotoxic lymphocytes (CD8+), NK cells (CD16+ CD56+ and T regulatory (T reg) lymphocytes (CD4+ CD25+). The different lymphocyte subsets were measured with a flow cytometric assay by using specific monoclonal antibodies supplied by Becton-Dickinson (Milan, Italy). Moreover, because of the importance not only of their absolute number, but also of their percentage with respect to the other lymphocyte subsets, namely to that of CD4+ cells, CD4/CD4CD25 ratio, corresponding to TH/T reg ratio, was also determined before and after therapy. Normal values (95% confidence limits) of T reg number and TH/T reg ratio observed in our laboratory were below 240/mm3 and above 4.0, respectively. Data were reported as mean +/- SE and statistically analyzed by the Student’s t test, the analysis of variance and the chi-square test, as appropriate.
III. Results
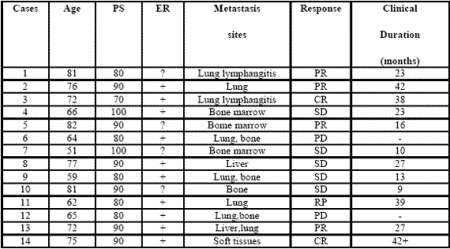
Evaluable patients were 22/24, while the remaining 2 patients, both affected by untreatable disseminated liver metastases due to colorectal cancer, rapidly died for disease progression before concluding the two planned months of arabinoxylan therapy. The clinical characteristics of the evaluable patients are reported in Table 1. Figure 1 illustrates changes in the mean number of total lymphocytes, T lymphocytes, T cytotoxic lymphocytes and NK cells occurring after 2 months of arabinoxylan therapy. No substantial variation was found in the mean number of lymphocytes, T lymphocytes, T cytotoxic lymphocytes and NK cells under arabinoxylan treatment. In contrast, as illustrated in Figure 2, TH and T reg mean numbers increased and decreased, respectively, after arabinoxylan therapy, without, however statistically significant differences with respect to the values seen prior to therapy. On the contrary, a statistically significant increase in TH/T reg mean ratio was achieved after arabinoxylan therapy (p<0.025). The increase in TH/T reg ratio under arabinoxylan therapy was more pronounced in patients with an abnormally low ratio prior to therapy with respect to that occurring in those with normal pre-treatment ratio, however without statistically significant differences ( 2.3 +/- 0.4 vs 1.7 +/- 0.5). In more detail,
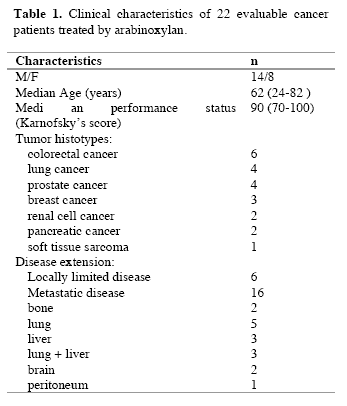

Figure 1. Changes in the number of lymphocytes, Tlymphocytes (CD3), T cytotoxic lymphocytes (CD8) and NK cells (CD16 CD56) after 2 months of arabinoxylan therapy.

Figure 2. Changes in the mean number of T helper (TH) lymphocytes (CD4) and T regulatory lymphocytes (cd4 cd24) and in TH/T reg mean ratio.
before arabinoxylan therapy, an abnormally low TH/T reg ratio was present in 12/22 (55%) evaluable patients. Arabinoxylan treatment induced a normalization of TH/T reg ratio in 5/12 (42%) patients with an abnormally low ratio prior to therapy. The percentage of arabinoxylan-induced TH/T reg normalization obtained in lymphocytopenic patients was not significantly different from that achieved in patients with normal pre-treatment lymphocyte count ( 3/7(43%) vs 2/5(40%) ). No toxicity was observed under arabinoxylan treatment, which was well tolerated in all patients. Asthenia was present in 8/22 (36%) evaluable patients. An evident relief of asthenia, as assessed by a specific patient report, was obtained under arabinoxylan therapy in 5/8 (63%) patients.
IV. Discussion
Previous experimental studies had already demonstrated some immunomodulating properties of arabinoxylan, in particular consisting of stimulation of NK cytotoxic function (Ghoneum, 1998), whereas NK cell number did not seem to be influenced by arabinoxylan administration. However, it has to be remarked that NK cells were belived to be fundamental in the antitumor immunity until some years ago, before the discovery of the essential role played by the antitumor cytokines, such as IL-2 and IL-12 (Whittington and Faulds, 1993) and dendritic cells, because of their function as antigen-presenting cells (Banks et al, 1995). In fact, it has to be considered that the cytotoxic activity of NK cells is effective only against artificial laboratory cancer cell lines, whose biological malignant properties are different from those presented by fresh human tumor cells (Whittington and Faulds, 1993). In addition, NK cells have been proven to be also able to destroy fresh human cancer cells only after the activation of their cytotoxic function by IL-2 (Atzpodien and Kirchner, 1990). From this point of view, arabinoxylan had been already proven to amplify the stimulatory effect of IL-2 on NK-mediated antitumor cytotoxicity (Ghoneum and Jewett, 2000). In contrast, no study has been performed up to now to evaluate the possible influence of arabinoxylan not only on the mechanisms responsible for the generation of an effective anticancer immune response, but also on those involved in the suppression of anticancer immunity. The results of this preliminary study, carried out to evaluate the influence of arabinoxylan on T reg cells, which represent the most important cells involved in the suppression of the antitumor cytotoxic immune response, demonstrates that arabinoxylan may counteract T reg cell generation by reducing their number and percentage with respect to the total amounts of CD4+ cells and circulating lymphocytes. Since NK cell function is inhibited by T reg activation (Shevach, 2002), the previously demonstrated arabinoxylan-induced stimulation of NK cell cytotoxic function might depend at least in part on its capacity of counteracting T reg generation (Dieckmann et al, 2001). Moreover, this study would suggest that the inhibitory action of arabinoxylan on T reg generation is more pronounced in patients with an abnormally high percentage of T reg cells prior to therapy, with a following pre-treatment abnormally low TH/T reg ratio before therapy, whereas its effect was less evident in patients with a pre-treatment value of TH/T reg ratio within the normal range. Therefore, the influence of arabinoxylan on T reg generation would consist of a modulatory action rather than an inhibitory activity. This finding could explain a potential favourable immunomodulatory effect of arabinoxylan also in patients with autoimmune diseases (Ghoneum, 1998), who in contrast to cancer patients would tend to present abnormally low amounts of T reg cells. In any case, the importance of the inhibition of T reg generation in the induction of an effective anticancer immune response has been recently confirmed by the evidence that the block of T reg activity by specific monoclonal antibodies may induce objective tumor regressions in humans (Yang et al, 2007). Obviously, the major problem is the exact identification of he T reg cell population. Even though T reg cells may express other immune markers, namely FOX-p2 cytoplasmatic antigen, most clinicians are in agreement to identify the CD4+CD25+ cells as T reg lymphocytes (12). In any case, further studies, by evaluating other immune markers, will be required to better identify T reg cells population, namely FOX-p3, even though recently some Authors have shown that FOX-p3 expression by T reg cells is associated with a lower suppressive activity (Dieckmann et al, 2001; Shevach, 2002). Moreover, it has to be remarked that several patients included in the present study were concomitantly under palliative therapy with the anti-cachectic pineal hormone melatonin (Brzezinski, 1997), which may also play immunomodulating effects (Maestroni, 1993). Therefore, further randomized studies with arabinoxylan alone versus arabinoxylan plus melatonin will be required to better define the immunomodulating action of arabinoxylan. If further clinical and experimental studies will confirm the inhibitory action of arabinoxylan on T reg cell system, it could be included in cytokine-based immunotherapies to enhance their efficacy by counteracting T reg cell generation.
References
Atzpodien J, Kirchner H (1990) Cancer, cytokines and cytotoxic cells:interleukin-2 in the immunotherapy of human neoplasms. Klin Wochenschr 14, 1-10.
Banks RE, Patel PM, Selby PJ (1995) Interleukin-12:a novel clinical player in cytokine therapy. Br J cancer 71, 655-659.
Brzezinski A (1997) Melatonin in humans. N Engl J Med 336, 185-195.
Dieckmann D, Plottner H, Berchtold S, Berger T, Schuler G (2001) Ex vivo isolation and characterization of CD4+CD25+ T cells with regulatory properties from human blood. J Exp Med 193, 1303-1310.
Ghoneum M (1998) Enhancement of human natural killer cell activity by modified arabinoxylane fro rice bran(MGN-3). Int J Immunother 14, 89-99.
Ghoneum M, Gollapudi S (2005) Synergistic of arabinoxilan rice bran (MGN-3/Biobran in S. Cerevisiae-induced apoptosis of monolayer breast cancer MFC-7 cells. Anticancer Res 25(6B), 4187-96.
Ghoneum M, Brown J, Gollapudi S (2007) Yeast therapy for the treatment of cancer and its enhancement by MGN-3/Biobran, an arabinoxylan rice bran. Cellular Signaling and Apoptosis Research (Ed. Alex R. Demasi) Cap IV: 185-200.
Ghoneum M, Jewett A (2000) Production of tumor necrosis factor-alpha and interferon-gamma from human peripheral blood lymphocytes by MGN-3, a modified arabinoxylan from rice bran, and its synergy with interleukin-2 in vitro. Cancer Detect Prevent 24, 314-324.
Lissoni P, Giani L, Zerbini S, Trabattoni P, Rovelli F (1998) Biotherapy with the pineal immunomodulating hormone melatonin versus melatonin plus Aloe vera in untreatable advanced solid neoplasms. Nat Immun 16, 27-33.
Maestroni JGM (1993) The immunoneuroendocrine role of melatonin. J Pineal Res 14, 1-10.
Markus J, Miller A, Smith M, Orengo I (2006) Metastatic hemangiopericytoma of the skin treated with wide local excision and MGN-3. Dermatol Surg 32, 145-147.
Matsuda T, Hirano T (1990) Interleukin-6 (IL-6). Biotherapy 2, 363-371.
Moore KW, O’Garra A, De Waal-Malefyt R (1993) Interleukin-10. Ann Rev Immunol 11, 165-174.
Rosenberg SA (1992) The immunotherapy and gene therapy of cancer. J Clin Oncol 10, 181-191.
Shevach EM (2002) CD4+CD25+ suppressor T cells:more questions than answers. Nat Rev Immunol 2, 389-400.
Whittington R, Faulds D (1993) Interleukin-2. Drugs 46, 446-514.
Yang JC, Hughes M, Kammula U, Royal R, Sherry RM, Topalian SL, Suri KB, Levy C, Allen T, Mavroukakis, Lowy I, White DE, Rosenberg SA (2007) Ipilimubab (anti-CTLA4 antibody)causes regression of metastatic renal cell cancer associated with enteritis and hypophysitis. J Immunother 30, 825-830.
(download PDF version)