Enhancement of the Efficacy of Chemotherapy with Oxaliplatin Plus 5-Fluorouracil by Pretreatment with IL-2 Subcutaneous Immunotherapy in Metastatic Colorectal Cancer Patients with Lymphocytopenia Prior to Therapy
Abstract. The present study was carried out to evaluate the influence of a short-period IL-2 administration on the efficacy of chemotherapy in rnetastatic colorectal cancer patients with pretreatment lymphocytopenia, which was defined as a lymphocyte count of less than 1500/mm3. The study included 144 consecutive metastatic colorectal cancer patients, who underwent chemotherapy with oxaliplatin plus 5-fluorouracil. Lymphocytopenia was seen in 4l/144 (28%) patients, who were randomized to receive chemotherapy alone or chemotherapy after a prechemoimmunotherapy with IL-2 (3 MIU twice/day for 3 consecutive days), whereas patients with a normal pretreatment lymphocyte count received only chemotherapy. A normalization of the lymphocyte number was achieved in 12/19 lymphocytopenic patients pretreated with IL-2.
The objective tumor regression rate achieved in patients with a normal lymphocyte count prior to chemotherapy was significantly higher compared to that obtained in lymphocytopenic patients treated with chemotherapy alone (54/103 vs. 3/22, p<0.01), whereas no significant difference occurred between patients with normal lymphocyte count and lymphocytopenic patients pretreated with IL-2 (54/103 vs. 8/19). This study confirms that pretreatment lymphocytopenia is associated with reduced efficacy of chemotherapy in metastatic colorectal cancer patients. Moreover, it suggests that pretreatment with IL-2 before the onset of chemotherapy may enhance the efficacy of chemotherapy in lymphocytopenic patients. Therefore, the administration of IL-2 before the onset of chemotherapy to improve the immune status of cancer patients may be considered as a new chemoimmunotherapeutic combination, which may be recommended in the treatment of advanced cancer patients, particularly in those with cancer-related immune alterations.
Despite great advances in the understanding of the immunobiology of tumors (1-3), their clinical management still remains founded on the biological characteristics of the tumor, namely of histology, stage, genetic profile and eventual expression of receptors for hormones and growth factors. Recent immunobiological discoveries have generally been limited to experimental studies. In particular, there is little data on the possible influence of the host immunobiological status on the efficacy of cancer chemotherapy for metastatic solid tumors.
However, preliminary clinical studies suggest that the evidence of lymphocytopenia, prior to the onset of chemotherapy, is associated with a reduced efficacy of the treatment, confirming the importance of the biological response of the patients in the prognosis of their neoplastic disease and in conditioning the efficacy of chemotherapy itself (4).
Previous clinical studies had already demonstrated that the occurrence of lymphocytopenia represents a negative biological prognostic factor, because of its association with a lower survival time (5). Cancer-related lymphocytopenia is the expression of the immunosuppressive status, which characterizes the progression of the neoplastic disease, and depends, at least in part, on the production of immunosuppressant substances by cancer cells (6). In particular, lymphocytopenia appears to be associated with a progressive decline in the blood concentrations of IL-2 (7), produced by T helper-type 1 lymphocytes (8). Since IL-2 represents the main growth factor for lymphocytes (9), at least from a theoretical point of view the lymphocytopenic status of advanced cancer patients could potentially be corrected and modified by the administration of IL-2 before starting the chemotherapeutic approach.
To date, several chemoimmunotherapeutic combinations with IL-2 plus chemotherapy have been proposed (10-12). However, all chemoimmunotherapeutic combinations so far elaborated have simply used the chemotherapeutic agents to induce damage to cancer cells, in an attempt to enhance their antigenicity, with the ensuing potential increased efficacy of IL-2 immunotherapy (10-12). However, there are no data about administering IL-2 in an attempt to enhance the efficacy of the commonly used chemotherapeutic regimens.
The present study was carried out to evaluate the influence of a short-period IL-2 subcutaneous (s.c.) immunotherapy on cancer-related lymphocytopenia, with the aim of increasing the efficacy of cancer chemotherapy in metastatic solid tumor patients.
Materials and Methods
The study included 144 metastatic colorectal cancer patients (M/F: 92/52; median age: years, range 41-75). The eligibility criteria were as follows: histologically proven metastatic colorectal cancer, measurable lesions, no double tumor, no brain metastases, no more than one previous chemotherapeutic line with 5-fluorouracil (5-FU) for the metastatic disease, and no concomitant chronic therapy with corticosteroids or other immunosuppressive drugs.
Lymphocytopenia was defined as the presence of a total lymphocyte number less than 1500/mm3 prior to the onset of chemotherapy and was observed in 41/144 (28%) patients prior to chemotherapy.
According to the metastatic locations, patients with pretreatment lymphocytopenia were randomized to receive chemotherapy alone, or to be treated with a short-period IL-2 s.c. immunotherapy before the onset of chemotherapy. The experimental protocol was explained to each patient and written consent was obtained.
According to the schedule proposed by Hochster et al. (13), the chemotherapeutic regimen consisted of oxaliplatin (OXA) plus 5-FU and folates. OXA was injected i. v. at 85 mg/m2 on days 1 and 15, while 5-FU and folates were administered i.v. at a dose of 500 mg/m2 and of 10 mg/m2 on days 1,8 and 15, thus representing a complete immunotherapeutic cycle.
The cycles were repeated every 28 days, for at least 3 cycles. In non-progressing patients, another 2 cycles were planned after the radiological examinations, including CT scan.
The prechemotherapeutic immunotherapy consisted of IL-2 at a dose of 3 million IU twice/daily for 3 consecutive days s.c., for a total dose of 18 million IU, during the week preceding the onset of chemotherapy.
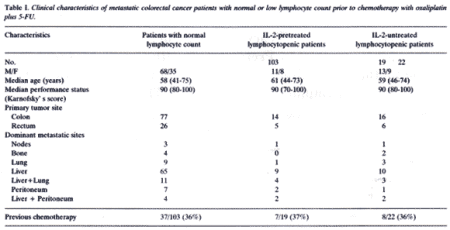
The clinical response was assessed according to WHO criteria. The clinical characteristics of the patients are reported in Table I. The data were statistically analyzed by the Chi-square test and the Student’s t-test, as appropriate.
Results
As shown in Table I , the three groups of patients with normal lymphocyte count prior to chemotherapy, or with pretreatment lymphocytopenia undergoing chemotherapy alone, or chemotherapy after an IL-2 prechemotherapeutic immunotherapy were well-matched and comparable for the main prognostic variables, including sites of metastases, location of the primary tumor, previous chemotherapies, age and performance status (PS), as assessed by Karnofsky’s score. The clinical response observed in the two groups of patients with normal or low lymphocyte count prior to chemotherapy is shown in Table II. A complete response (CR) was achieved in 5/103 (5%) patients with normal lymphocyte count prior to therapy and in only 1/41 (2%) lymphocytopenic patients. A partial response (PR) was achieved in 59/144 patients, while the overall response rate (CR + PR) was 65/144 (45%). The response rate (CR + PR) achieved in patients with normal lymphocyte count prior to therapy was significantly higher than that found in patients with pretreatment lymphocytopenia (54/103 (52%) vs. 11/41 (27%), p<0.05).
A normalization of lymphocyte number prior to therapy, with values greater than 1500/mm3, was reached in 12/19 patients in response to the prechemotherapeutic immunotherapy with IL-2.
On considering the influence of IL-2 immunotherapy on the clinical response to chemotherapy, the response rate obtained in patients with a normal lymphocyte count was significantly higher compared to that observed in lymphocytopenic patients, who received no immunotherapy prior to chemotherapy (54/103 vs. 3/22, p<0.01), whereas no difference was seen in the response rate between patients with a normal lymphocyte count prior to therapy and lymphocytopenic patients pretreated with IL-2 (54/103 vs. 8/19).
Finally, within the group of lymphocytopenic patients pretreated with IL-2 before the onset of chemotherapy, the response rate observed in patients who achieved a normalization of lymphocyte count in response to IL-2 injection was higher with respect to that obtained in patients whose lymphocyte number was not normalized by IL-2 (6/12 (50% ) vs. 2/7 (29%)), even though the difference was not statistically significant. However, the response rate obtained in IL-2-pretreated patients, even independently of lymphocyte normalization, was significantly higher than that found in lymphocytopenic patients without IL-2 immunotherapy (8/19 vs. 3/22,p<0.05).
IL-2 prechemotherapeutic immunotherapy was well tolerated and did not influence the expected toxicity of the chemotherapy.

Discussion
In accordance with previous preliminary clinical results (4), this study confirms that pretreatment lymphocytopenia is associated with a reduced efficacy of chemotherapy in metastatic cancer patients. This finding is not surprising, since lymphocyte activation would contribute to the destruction of tumor cells induced by chemotherapy, particularly of chemotherapy-resistant cells, which are more sensitive to the cytotoxicity exerted by activated lymphocytes (10). In the presence of lymphocytopenia, such participation by lymphocytes would be diminished.
The results of this study demonstrated that the correction of lymphocytopenia by the administration of the main growth factor for lymphocytes, IL-2, may allow enhanced efficacy of the chemotherapy, comparable to that commonly observed in patients with a normal lymphocyte count prior to chemotherapy. Thus, a new regimen for immunochemotherapeutic strategies, consisting of the administration of in vivo 19: 1077-1080 (2005)
antitumor cytokines, such as IL-2, before the onset of chemotherapy, should improve the immune status of the patients.
At present, the overall chemoimmununotherapeutic regimens with IL-2 plus chemotherapy have utilized chemotherapy to improve the efficacy of IL-2, by enhancing the antigenicity of cancer cells as a consequence of chemotherapy-induced damage of cell surface molecules. In contrast, this study would suggest the possibility of using IL-2 to enhance the efficacy of chemotherapy, by improving the immune status of cancer patients, namely by increasing the absolute number of lymphocytes, which play a fundamental role in mediating an effective anticancer immune reaction.
Further studies, using different doses of IL-2 and for a longer period of injection, will be required to better define the optimal schedule to prepare cancer patients for the successive administration of chemotherapy.
References
Atzpodien J and Kirchner H: Cancer, cytokines and cytotoxic cells: interleukin-2 in the immunotherapy of human neoplasms. Klin Wochenschr 68: 1-11, 1990.
Rosenberg SA: The immunotherapy and gene therapy of cancer. J Clin Oncol 10: 180-199, 1992.
Whittington R and Faulds D: Interlerleukin-2. Drugs 46: 446-483.
Lissoni P, Brivio F, Fumagalli L et al: Efficacy of cancer chemotherapy in relation to the pretreatment number of lymphocytes in patients with metastatic solid tumors. Int J Biol Markers 19: 135-140, 2004.
Riesco A: Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. Cancer 25: 135-140, 1970.
Fumagalli L, Lissoni P, Di Felice G et al: Pretreatment serum markers and lymphocyte response to interleukin-2 therapy. Br J Cancer 80: 407-411, 1999.
Lissom P, Barni S, Rovelli F and Tancini G: Lower survival in metastatic cancer patients with reduced interleukin-2 blood concentrations. Oncology 48: 125-127, 1991.
Grimm EA, Mazumder A, Zhang HZ and Rosenberrg SA: Lymphokine-activated killer cell phenomenon. J Exp Med 155: 1823-1841, 1982.
Herberman RB: Design of clinical trials with biological response modifiers. Cancer Treat Rep 69: 1161-1164, 1985.
Tartour E, Blay JY, Dorval T et al: Predictors of clinical response to interleukin-2-based immunotherapy in melanoma patients. J Clin Oncol 14: 1697-1703, 1996.
Bernengo MG, Quaglino O, Cappelio N et al: Macrophagemediated immunostimulation modulates therapeutic efficacy of interleukin-2-based chemoimmunotherapy in advanced metastatic melanoma patients. Melanoma Res 10: 55-65, 2000.
Lissom P, Vaghi M, Ardizzoia A et al: A phase II study of chemoneuroimmunotherapy with platinum, subcutaneous lowdose interleukin-2 and the pineal neurohormone melatonin (P.I.M.) as a second-line therapy in metastatic melanoma patients progressing on dacarbazine plus interferon-alpha. In Vivo 16: 93-96, 2002.
Hochster H, Charchowa A, Speyer J et al: Oxaliplatin with weekly bolus fluorouracil and low-dose leucovorin as first-line therapy for patients with colorectal cancer. J Clin Oncol 21: 2703-2707, 2003.
Correspondence to: Dr Paolo Lissoni, Divisione di Radioterapia Oncologica, Ospedale San Gerardo, 20052 Monza, Milano, Italy. Fax: 039/2332284.
Key Words: Immunotherapy, interleukin-2, lymphocytopenia, oxaliplatin.
Received April 7, 2005 Accepted August 30, 2005
P. LISSONI1, F. BRIVIO2, L. FUMAGALLI2, G. DI FEDE3 and G. BRERA4
1Division of Radiation Oncology and 2Division of Surgery, S. Gerardo Hospital, Monza, Milan; 3Institute of Biological Medicine, Milan; 4Ambrosian University, Milan, Italy





